Yesterday's graph is wrong
I awoke early this morning with a nagging feeling that I had made a bad graph yesterday. The second graph in "Musing on Sxy", which outlines my predictions about how adding cAMP to log-phase cells will affect transformation frequency, is missing data we already have. What would help is if my imaginary graph became more concrete with some actual numbers and data (again from Redfield 1991). First, a reminder that our simple model for the regulation of competence currently involves two activators (or two “on” switches), CRP and Sxy. CRP levels are fairly constant throughout different growth stages; by supplying 1mM of it’s allosteric effector cAMP, we are turning that switch full on.
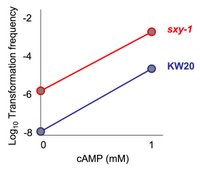 This graph is now based on real data - the four data points are roughly the average transormation frequencies measured in Redfield 1991. Here we see moderate competence development in KW20 when CRP is fully activated, even though KW20 has very little Sxy during log-phase growth. Thus even very low levels of Sxy are sufficient to allow some competence, in contrats to what I said yesterday. Also, this helps clarify what I was already planning for today’s blog: I want to take a reciprocal approach to yesterday and look at what limits maximal competence when both the CRP and Sxy switches are turned on. (A subsequent blog will discuss the role of stochasticity and competition/interference between regulators - but that’s trickier and needs clear groundwork thinking. I’ll stick with the simple on/off switch analogy for now).
This graph is now based on real data - the four data points are roughly the average transormation frequencies measured in Redfield 1991. Here we see moderate competence development in KW20 when CRP is fully activated, even though KW20 has very little Sxy during log-phase growth. Thus even very low levels of Sxy are sufficient to allow some competence, in contrats to what I said yesterday. Also, this helps clarify what I was already planning for today’s blog: I want to take a reciprocal approach to yesterday and look at what limits maximal competence when both the CRP and Sxy switches are turned on. (A subsequent blog will discuss the role of stochasticity and competition/interference between regulators - but that’s trickier and needs clear groundwork thinking. I’ll stick with the simple on/off switch analogy for now). Maximal competence (ie. a transformation frequency just over 0.01 with good, fresh transforming DNA) develops when cells are incubated in MIV medium for 90 minutes. Redfield 1991 found that the sxy-1 hypercompetent mutant achieves the same high transformation frequency in MIV and during late log-phase growth. When I repeated this experiment to test all hypercompetent mutants, I observed a ~5 fold lower maximal transformation frequency in MIV , and transformation frequency in late log was 7-10 fold lower again (see graph).
 However, I used old transforming DNA and found that with fresh DNA, transformation frequencies in MIV were the same as Redfield 1991 (not shown on graph. Also, I did not test late log growth with fresh DNA). This raises the possibility that in Redfield 1991, using very potent, fresh transforming DNA obscured a difference in transformability between MIV and late log conditions, which only became apparent when using old (sub-saturating?) DNA. If so, why don’t hypercompetent sxy mutants achieve maximal transformation in late log-phase? One possibility is that the cells are maximally competent, but the transformation machinery within cells is more efficient in MIV conditions. However, I will explore the alternate hypothesis that hypercompetent sxy mutants are not maximally competent in late log growth.
However, I used old transforming DNA and found that with fresh DNA, transformation frequencies in MIV were the same as Redfield 1991 (not shown on graph. Also, I did not test late log growth with fresh DNA). This raises the possibility that in Redfield 1991, using very potent, fresh transforming DNA obscured a difference in transformability between MIV and late log conditions, which only became apparent when using old (sub-saturating?) DNA. If so, why don’t hypercompetent sxy mutants achieve maximal transformation in late log-phase? One possibility is that the cells are maximally competent, but the transformation machinery within cells is more efficient in MIV conditions. However, I will explore the alternate hypothesis that hypercompetent sxy mutants are not maximally competent in late log growth. Hypercompetent sxy mutants in late log-phase have equal amounts or more Sxy than does KW20 when it is maximally competent in MIV, therefore Sxy is not limiting. The cAMP data described yesterday and at the beginning of this post shows that cAMP is not limiting. Thus, I can elaborate the first graph, and include a possible role for a third regulator.
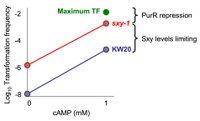 We have good evidence that PurR, a protein that represses gene expression in the presence of the purine bases hypoxanthine or guanine, is a third regulator of competence. To date, we have been testing for PurR activity by supplementing growth media with purine nucleotides, but this has been problematic partly because cells don’t like having high levels of these nucleotides in their growth medium. If all of my speculation presented here has some validity, we can test for PurR activity in late log-phase by measuring transformation frequency in double hypercompetent sxy/purR- mutants. If the hypothesis is correct, then when transforming with sub-saturating DNA, hypercompetent purR- cells will have higher transformation frequencies than their hypercompetent parents.
We have good evidence that PurR, a protein that represses gene expression in the presence of the purine bases hypoxanthine or guanine, is a third regulator of competence. To date, we have been testing for PurR activity by supplementing growth media with purine nucleotides, but this has been problematic partly because cells don’t like having high levels of these nucleotides in their growth medium. If all of my speculation presented here has some validity, we can test for PurR activity in late log-phase by measuring transformation frequency in double hypercompetent sxy/purR- mutants. If the hypothesis is correct, then when transforming with sub-saturating DNA, hypercompetent purR- cells will have higher transformation frequencies than their hypercompetent parents.

1 Comments:
You are so hardworking up there!! hope you are having tons of fun too!
Post a Comment
<< Home